We are a biophysics group covering three levels of research: Microscopy method development, membrane biophysics and immunology.
Microscopy method development:
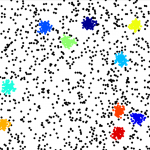
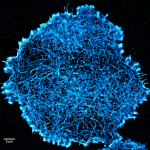
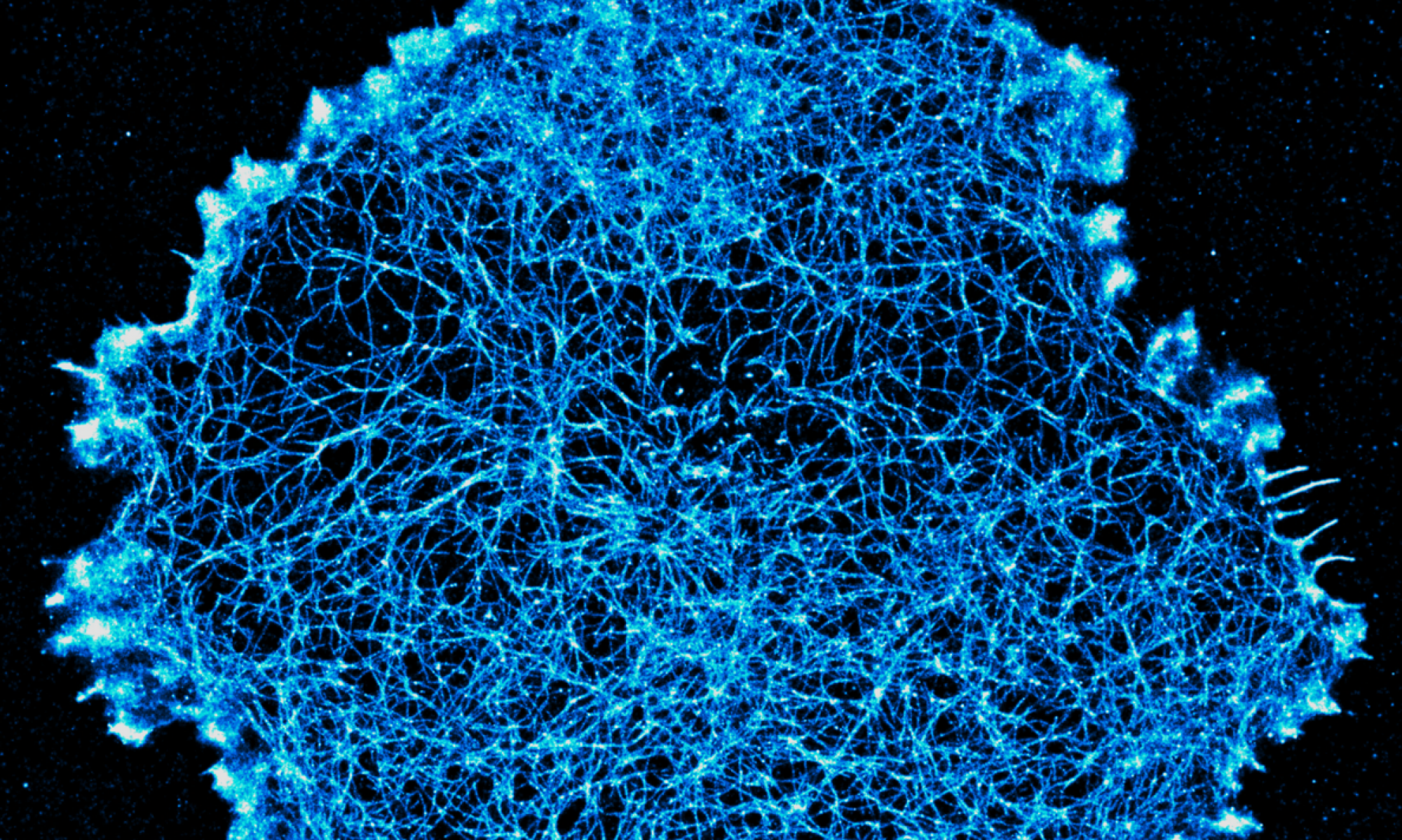
We work to develop new fluorescence microscopy tools designed to study the plasma membrane and related biophysical phenomena. In particular, we develop post-processing analytics for single-molecule localisation microscopy (SMLM) data, whose output is in the form of a spatial point pattern, rather than a traditional pixelated image. In particular, we have developed a variety of cluster analysis methods for the quantitative description of molecular spatial organisation. This includes nanoscale clustering in 2D and 3D, live-cell cluster analysis and multi-colour co-cluster analysis. We have also developed algorithms to extract descriptions of fibrous patterns, such as those derived from components of the cytoskeleton. Finally, we also employ environmentally-sensitive fluorescent probes which report on their local biophysical and biochemical environments. These probes are combined with multi-channel, spectral and lifetime detection.
Membrane biophysics:
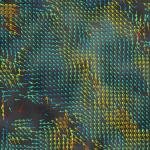
We are interested in quantitatively describing the structure of biological membranes, especially the plasma membrane in mammalian cells. Here, we investigate a) the properties of membrane lipid microdomains (the lipid raft hypothesis) b) the nanoscale spatio-temporal organisation of membrane proteins (e.g. nanoscale protein clustering) and c) the structure of, and interactions with, the cortical actin cytoskeletal meshwork. We seek to develop new methodology to analyse these phenomena, to 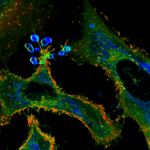 determine on what these organisational elements depend, and how they affect cell outcomes, in particular cell signalling. To do this, we primarily use advanced fluorescence imaging, biochemical methods, MD simulations and systems biology approaches.
determine on what these organisational elements depend, and how they affect cell outcomes, in particular cell signalling. To do this, we primarily use advanced fluorescence imaging, biochemical methods, MD simulations and systems biology approaches.
Immunology:
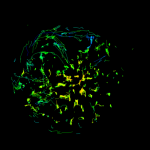 Our main biological application is in T cells. T cell activation must be finely regulated as failure to activate can lead to pathogen-linked disease and inappropriate activation can lead to autoimmune disease. Much of this regulation is dependent on the spatio-temporal organisation of signalling as regulated by membrane biophysical phenomena. Specifically, we study the molecular organisation at the T cell immunological synapse and during T cell migration and are particularly interested in the T cell receptor (TCR) itself, LAT, Lck and ZAP70, integrins (LFA-1) and the Lyp-Csk-PAG complex as well as the structure and function of cortical actin.
Our main biological application is in T cells. T cell activation must be finely regulated as failure to activate can lead to pathogen-linked disease and inappropriate activation can lead to autoimmune disease. Much of this regulation is dependent on the spatio-temporal organisation of signalling as regulated by membrane biophysical phenomena. Specifically, we study the molecular organisation at the T cell immunological synapse and during T cell migration and are particularly interested in the T cell receptor (TCR) itself, LAT, Lck and ZAP70, integrins (LFA-1) and the Lyp-Csk-PAG complex as well as the structure and function of cortical actin.